Inspection at NJ Facility Concludes
The inspection was carried out by the regulatory body from September 29 to October 10, 2025.
In the exchange filing, the company also added that it 'will address the observation and respond to the FDA within the stipulated timeframe'.
Lupin also added that the Mumbai-based pharma company is 'committed to being compliant with CGMP standards across all our facilities.
The company was in the news earlier this week, for another development from the United States, after on October 9, the company announced that it has plans to establish a state-of-the-art pharmaceutical manufacturing facility with an investment of $250 million. This facility is slated to come up in Coral Springs, Florida.
This facility will look to enhance the US-based production of critical respiratory medicines. The company will invest the aforementioned $250 million over the period of 5 years.
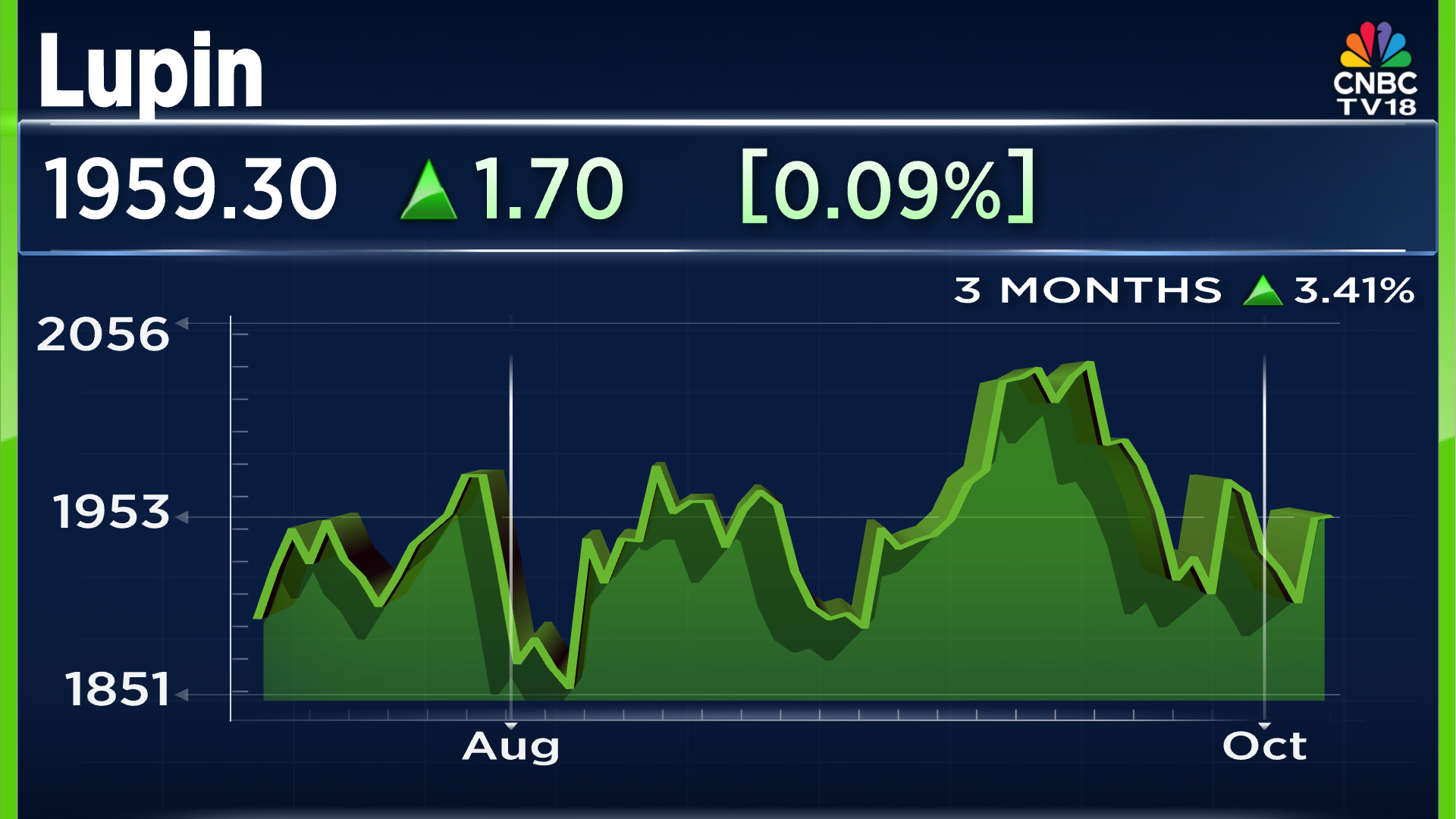
When we take a look at the company stocks, on the last day of trade before closing for the week, the stock closed in green with negligible gains of 0,09% or ₹1.70. However, in the past three months of trade, the company's shares have seen an uptick of 3.41% in their value. The current share price stands at ₹1,959 per share.
Read Also: Lupin shares surge over 3% on $250 million investment plans for new US facility
/images/ppid_59c68470-image-176017002952860357.webp)








/images/ppid_a911dc6a-image-177061642411946959.webp)




