What's Happening?
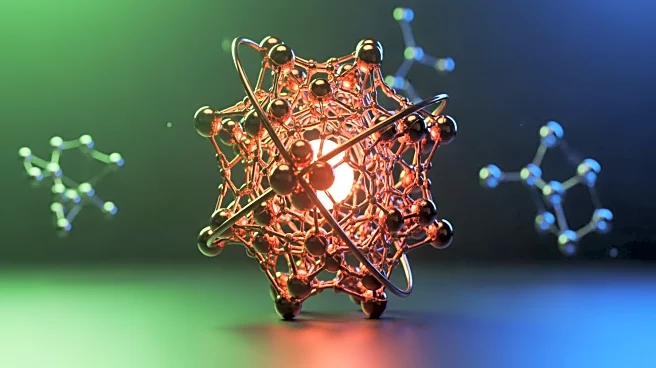
Researchers from Tsinghua University in Beijing have successfully created a stable superatom composed of 45 copper atoms, a significant advancement in chemical engineering. This superatom, known as Cu45, is designed to behave like a single atom but with enhanced stability, similar to noble gases. The innovation lies in its ability to withstand harsh conditions that typically degrade copper, such as exposure to boiling water, concentrated acids, and high temperatures. The superatom is encased in a protective shell of organic molecules, preventing it from disintegrating. This breakthrough allows the superatom to act as an efficient catalyst in converting carbon dioxide into ethylene, achieving over 80% efficiency. Ethylene is a crucial industrial
chemical used in the production of plastics and fuels.
Why It's Important?
The development of the Cu45 superatom represents a potential game-changer in carbon recycling and industrial chemistry. By providing a stable and efficient method to convert carbon dioxide into valuable chemicals like ethylene, this technology could significantly reduce carbon emissions and contribute to more sustainable industrial processes. The ability to use copper, a relatively inexpensive and abundant metal, enhances the economic feasibility of this approach. This advancement could lead to broader applications in electrocatalysis, offering new pathways for reducing the environmental impact of industrial activities and supporting the transition to greener technologies.
What's Next?
The research team anticipates that their discovery will pave the way for the development of more robust copper-based catalysts for a variety of electrocatalytic applications. Future research will likely focus on optimizing the design and application of these nanoclusters to enhance their performance and expand their use in different industrial processes. The potential for scaling up this technology could attract interest from industries looking to improve their carbon footprint and adopt more sustainable practices. Continued exploration in this field may also lead to new insights into the design of other metal-based superatoms for various catalytic applications.