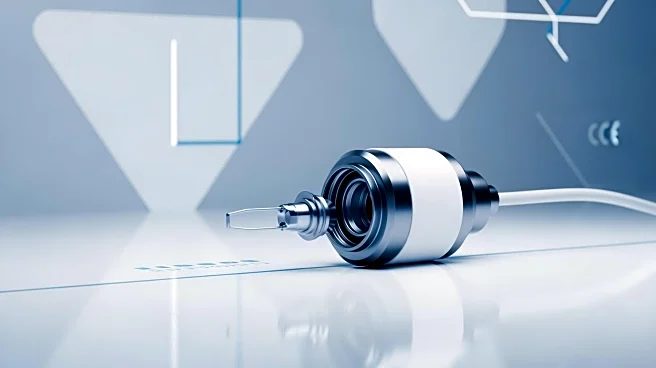
What's Happening?
Acarix has received EU MDR certification for its CADScor System, a non-invasive diagnostic tool for ruling out significant coronary artery disease. This certification confirms the system's compliance with stringent EU safety and quality standards, facilitating its expanded use across European markets. The CADScor System, which uses acoustics and computational processing to analyze coronary blood flow, aims to reduce unnecessary diagnostic procedures. Acarix plans to leverage this certification to enhance its global market presence, including in the U.S., where efficient diagnostic solutions are in high demand.
Why It's Important?
The EU MDR certification is a critical milestone for Acarix, enabling the company to expand its market reach and enhance its competitive
position in the medical device industry. The CADScor System's ability to provide rapid, non-invasive diagnostics aligns with healthcare priorities to improve patient care and reduce costs associated with unnecessary testing. This development could lead to broader adoption of the technology in the U.S., where healthcare systems are increasingly focused on efficient and evidence-based diagnostic tools. The certification also reinforces Acarix's commitment to maintaining high regulatory standards.