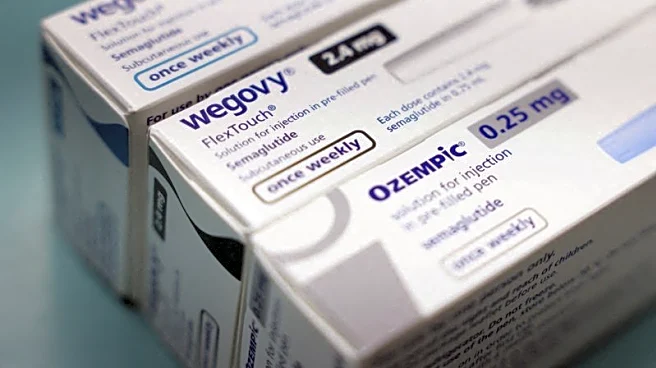
What's Happening?
Manufacturers of GLP-1 weight-loss drugs, including Novo Nordisk and Eli Lilly, are facing over 4,400 lawsuits in the U.S. due to alleged severe side effects not listed on the drugs' labels. These side effects include sudden blindness, severe vision changes, and gastroparesis, a condition that affects the stomach's ability to empty food. The lawsuits represent a small fraction of the 31 million Americans using these drugs. Despite the legal challenges, the companies maintain that their products are safe and plan to defend against the claims. The increasing use of these drugs has also impacted consumer buying habits, particularly in the grocery and convenience-store sectors.
Why It's Important?
The lawsuits against GLP-1 drug manufacturers highlight significant concerns
about drug safety and transparency in labeling potential side effects. This legal battle could have substantial implications for the pharmaceutical industry, potentially leading to stricter regulations and more comprehensive safety disclosures. Additionally, the outcome of these cases may influence consumer trust and the future market dynamics of weight-loss medications. The situation underscores the need for ongoing monitoring and evaluation of drug safety to protect public health.