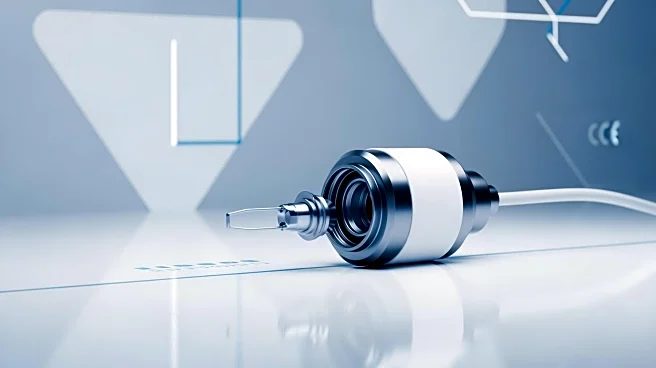
What's Happening?
MethodSense, a regulatory and quality consulting firm, has successfully supported ulrich medical in obtaining its fourth FDA clearance for the CT Motion Injector, a next-generation syringe-less injector
system used in diagnostic imaging. This clearance marks a significant milestone in the partnership between MethodSense and ulrich medical, which began in 2012. The CT Motion Injector introduces several advancements, including extended usability of disposable components, increased pharmaceutical capacity, and enhanced compatibility with various contrast media. These improvements aim to boost clinical efficiency, reduce patient wait times, and enhance safety in radiology departments.
Why It's Important?
The FDA clearance of ulrich medical's CT Motion Injector is crucial for the medical imaging industry, as it promises to improve workflow efficiency and patient safety in radiology departments. By allowing extended use of disposable components and supporting a higher capacity of contrast media, the system reduces setup time and consumable waste, which can lead to cost savings for healthcare facilities. Additionally, the system's compatibility with multiple pharmaceutical partners and its automated contrast medium density adjustment feature enhance its versatility and accuracy, potentially leading to better patient outcomes and lower operational costs.
What's Next?
Following this FDA clearance, ulrich medical is poised to expand its market presence in the U.S. The company, with the support of MethodSense, plans to continue its growth trajectory by leveraging the enhanced features of the CT Motion Injector to attract more healthcare providers. The focus will likely be on demonstrating the system's efficiency and safety benefits to radiology departments across the country. As ulrich medical continues to innovate and expand its product portfolio, it may seek further regulatory approvals to introduce additional advancements in medical imaging technology.