What is the story about?
Drug firm Lupin Ltd on Wednesday (February 4) announced the launch of Topiramate extended-release capsules in the United States in 25 mg, 50 mg, 100 mg, and 200 mg strengths, following approval of its Abbreviated New Drug Application (ANDA) by the US FDA.
The capsules are bioequivalent to the reference listed drug, Trokendi XR extended-release capsules of Supernus Pharmaceuticals, Inc. They are indicated as initial monotherapy for partial-onset or primary generalised tonic-clonic seizures in patients aged six years and older.
They can also be used as adjunctive therapy for partial-onset seizures, primary generalised tonic-clonic seizures, and seizures associated with Lennox-Gastaut syndrome in patients aged six years and above, as well as for preventive treatment of migraine in patients aged 12 years and older.
Also Read: Lupin shares gain ahead of US drug launch that can fetch $200 million in FY26
The US market for Topiramate extended-release capsules is estimated at $164 million annually, according to IQVIA MAT December 2025 data.
This week, Lupin Ltd announced two key developments spanning its innovation and generics businesses, including a tuberculosis-focused drug collaboration and the launch of an oncology generic in the US.
Lupin said it has entered a strategic collaboration with TB Alliance to advance the clinical development and commercialisation of Telacebec, an investigational drug for the treatment of tuberculosis (TB) and other mycobacterial diseases, including leprosy and Buruli ulcer.
Under the agreement, TB Alliance will continue to lead the clinical development of Telacebec, while Lupin will support the programme through its global manufacturing, regulatory and supply chain capabilities to enable wider patient access once approved.
Also Read: Lupin shares in focus after signing license and supply agreement for Semaglutide in 23 countries
Telacebec, formerly known as Q203, is being developed as a novel therapy for TB and related infections that continue to affect millions of people globally, particularly in low- and middle-income countries.
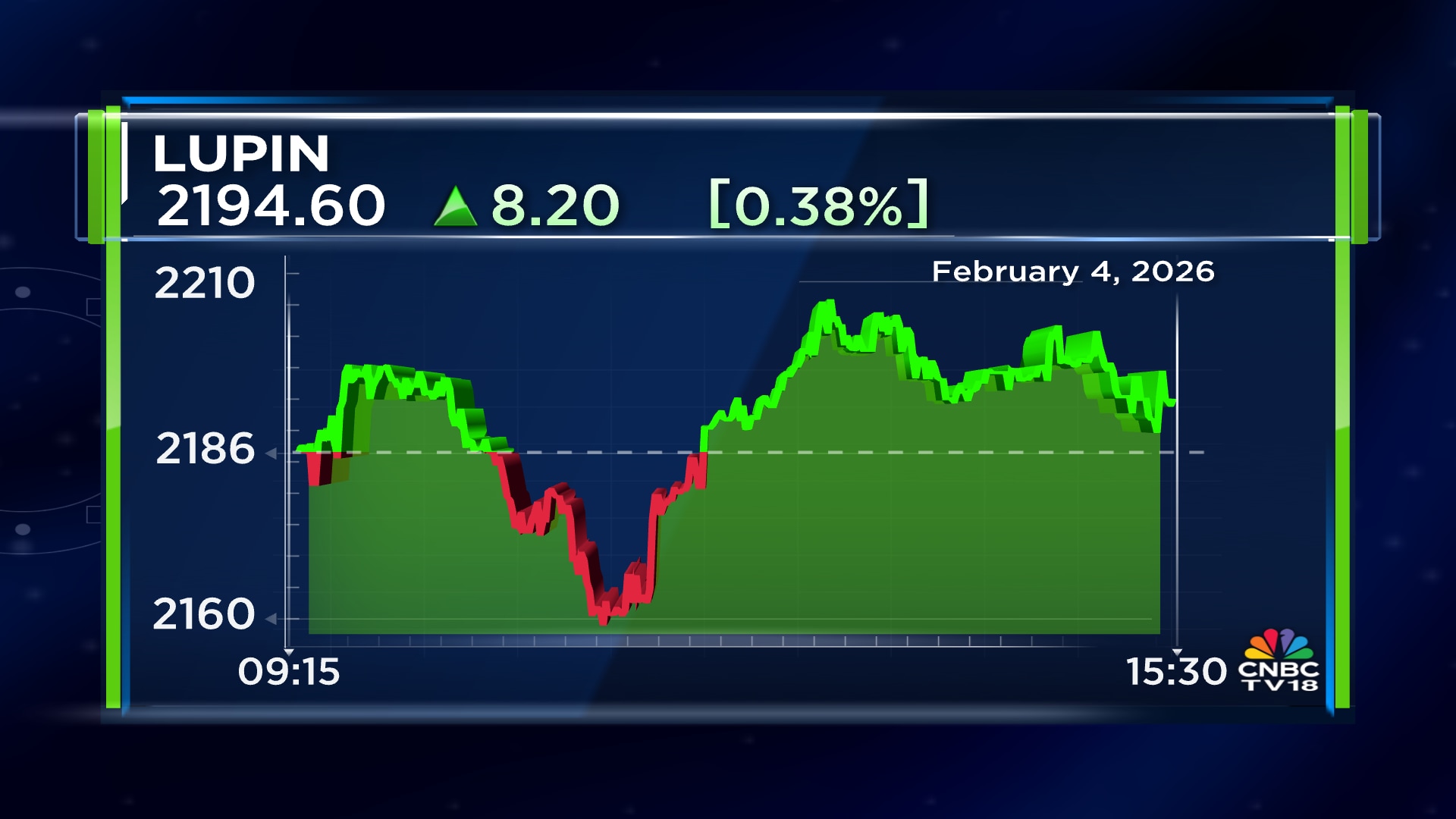
Shares of Lupin Ltd ended at ₹2,195.00, up by ₹8.60, or 0.39%, on the BSE.
The capsules are bioequivalent to the reference listed drug, Trokendi XR extended-release capsules of Supernus Pharmaceuticals, Inc. They are indicated as initial monotherapy for partial-onset or primary generalised tonic-clonic seizures in patients aged six years and older.

They can also be used as adjunctive therapy for partial-onset seizures, primary generalised tonic-clonic seizures, and seizures associated with Lennox-Gastaut syndrome in patients aged six years and above, as well as for preventive treatment of migraine in patients aged 12 years and older.
Also Read: Lupin shares gain ahead of US drug launch that can fetch $200 million in FY26
The US market for Topiramate extended-release capsules is estimated at $164 million annually, according to IQVIA MAT December 2025 data.
This week, Lupin Ltd announced two key developments spanning its innovation and generics businesses, including a tuberculosis-focused drug collaboration and the launch of an oncology generic in the US.
Lupin said it has entered a strategic collaboration with TB Alliance to advance the clinical development and commercialisation of Telacebec, an investigational drug for the treatment of tuberculosis (TB) and other mycobacterial diseases, including leprosy and Buruli ulcer.
Under the agreement, TB Alliance will continue to lead the clinical development of Telacebec, while Lupin will support the programme through its global manufacturing, regulatory and supply chain capabilities to enable wider patient access once approved.
Also Read: Lupin shares in focus after signing license and supply agreement for Semaglutide in 23 countries
Telacebec, formerly known as Q203, is being developed as a novel therapy for TB and related infections that continue to affect millions of people globally, particularly in low- and middle-income countries.
Shares of Lupin Ltd ended at ₹2,195.00, up by ₹8.60, or 0.39%, on the BSE.
/images/ppid_59c68470-image-17702300304481188.webp)

/images/ppid_59c68470-image-177023253182623149.webp)
/images/ppid_a911dc6a-image-177023093278372458.webp)
/images/ppid_a911dc6a-image-177023086790995091.webp)


/images/ppid_a911dc6a-image-17702370303737089.webp)









/images/ppid_a911dc6a-image-177023352404224347.webp)
/images/ppid_a911dc6a-image-177023343772090560.webp)
