What is the story about?
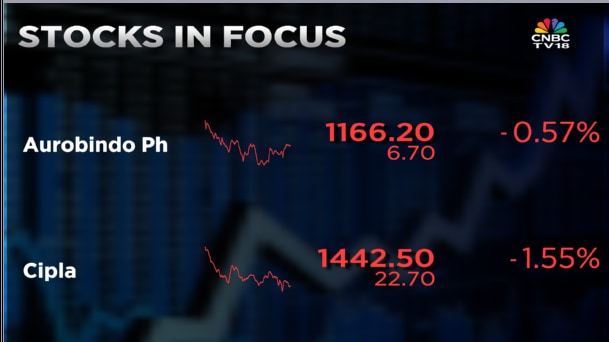
Shares of Aurobindo Pharma and Cipla are in focus on Tuesday, January 13, after Respirent Pharmaceuticals and Lannett received final approval from the US Food and Drug Administration for a generic version of Advair Diskus.
Advair is a key respiratory drug used to treat asthma and chronic obstructive pulmonary disease.
Respirent received final approval for its ANDA for fluticasone propionate and salmeterol, the generic equivalent of Advair Diskus.
The approval is huge as Aurobindo Pharma had announced the acquisition of Lannett in July 2025 for $250 million. While the transaction is yet to be completed, analysts expect the acquisition to close within the next two months.
According to analysts, if the acquisition goes through and includes this newly approved product, it could be a positive development for Aurobindo Pharma.
The development, however, is being seen as negative for Cipla.
Cipla had filed its own generic version of Advair as early as 2020 and has been awaiting final approval.
Street estimates suggest Cipla could generate peak sales of around $100 million from its Advair generic, with a potential launch expected in the second half of FY27.
With Mylan, now Viatris, having already received approval for its generic Advair in 2019, the latest approval for the Aurobindo Pharma-linked entity adds to competition in the market.
According to brokerage firm Nuvama, the increased competitive intensity following this approval is negative for Cipla, as it raises the risk to market share and peak sales expectations for its Advair inhaler opportunity.
Advair is a key respiratory drug used to treat asthma and chronic obstructive pulmonary disease.

Respirent received final approval for its ANDA for fluticasone propionate and salmeterol, the generic equivalent of Advair Diskus.
The approval is huge as Aurobindo Pharma had announced the acquisition of Lannett in July 2025 for $250 million. While the transaction is yet to be completed, analysts expect the acquisition to close within the next two months.
According to analysts, if the acquisition goes through and includes this newly approved product, it could be a positive development for Aurobindo Pharma.
The development, however, is being seen as negative for Cipla.
Cipla had filed its own generic version of Advair as early as 2020 and has been awaiting final approval.
Street estimates suggest Cipla could generate peak sales of around $100 million from its Advair generic, with a potential launch expected in the second half of FY27.
With Mylan, now Viatris, having already received approval for its generic Advair in 2019, the latest approval for the Aurobindo Pharma-linked entity adds to competition in the market.
According to brokerage firm Nuvama, the increased competitive intensity following this approval is negative for Cipla, as it raises the risk to market share and peak sales expectations for its Advair inhaler opportunity.
/images/ppid_59c68470-image-176828502846982058.webp)

/images/ppid_59c68470-image-177071008324940804.webp)
/images/ppid_59c68470-image-177071015030845145.webp)
/images/ppid_59c68470-image-17707051657521865.webp)
/images/ppid_59c68470-image-177096007386712864.webp)
/images/ppid_59c68470-image-177095508098299086.webp)
/images/ppid_59c68470-image-177078007003694796.webp)
/images/ppid_59c68470-image-177069774045880142.webp)
/images/ppid_59c68470-image-17706926650825720.webp)
/images/ppid_59c68470-image-177070527012744285.webp)
/images/ppid_59c68470-image-177070016688485506.webp)
/images/ppid_59c68470-image-177070275456679704.webp)
/images/ppid_59c68470-image-177069782473547371.webp)