What is the story about?
Lupin has announced that the United States Food and Drug Administration's inspection of its Nagpur facility has come to a close.
In an exchange filing published on November 15, the pharma company said that the drug regulator concluded the inspection of its Nagpur Unit-1 Facility with "No Observations”.
This inspection from the USFDA was carried out as a part of a PreApproval Inspection at its Unit-1 oral solid dosage manufacturing facility.
This inspection closed with zero FDA 483 observations.
Here, the FDA's 483 observation is a notice issued by the US-based regulatory body's inspector to a pharma company after an inspection, listing any potential regulatory violations found at the manufacturing facility.
The inspection from officials lasted for 4 days; it was carried out from November 10 to November 14, 2025.
Talking about the 'successful outcome', Nilesh Gupta, Managing Director, Lupin, said, “The successful outcome of the US FDA inspection at our Nagpur Unit-1 facility exemplifies our commitment to uphold and maintain the highest standards of quality, compliance, and safety across our facilities. We remain dedicated to improving the lives of our patients globally.”
When we take a look at the company's recent performance, in its Q2FY26 results, the company reported a 73.34% year-on-year surge in net profit to ₹1,478 crore for Q2.
Revenue from operations grew 24.2% year-on-year to ₹7,047.5 crore. When we look at other crucial facets, the Earnings before interest, tax, depreciation, and amortisation (EBITDA) saw a rise of 74.7% to ₹2,341.7 crore, against ₹1,340.5 crore in the previous cycle.
Furthermore, when we gauge the profitability, the company's margin grew to 33.2%, a near 100 basis point rise from the previous year's 23.6%.
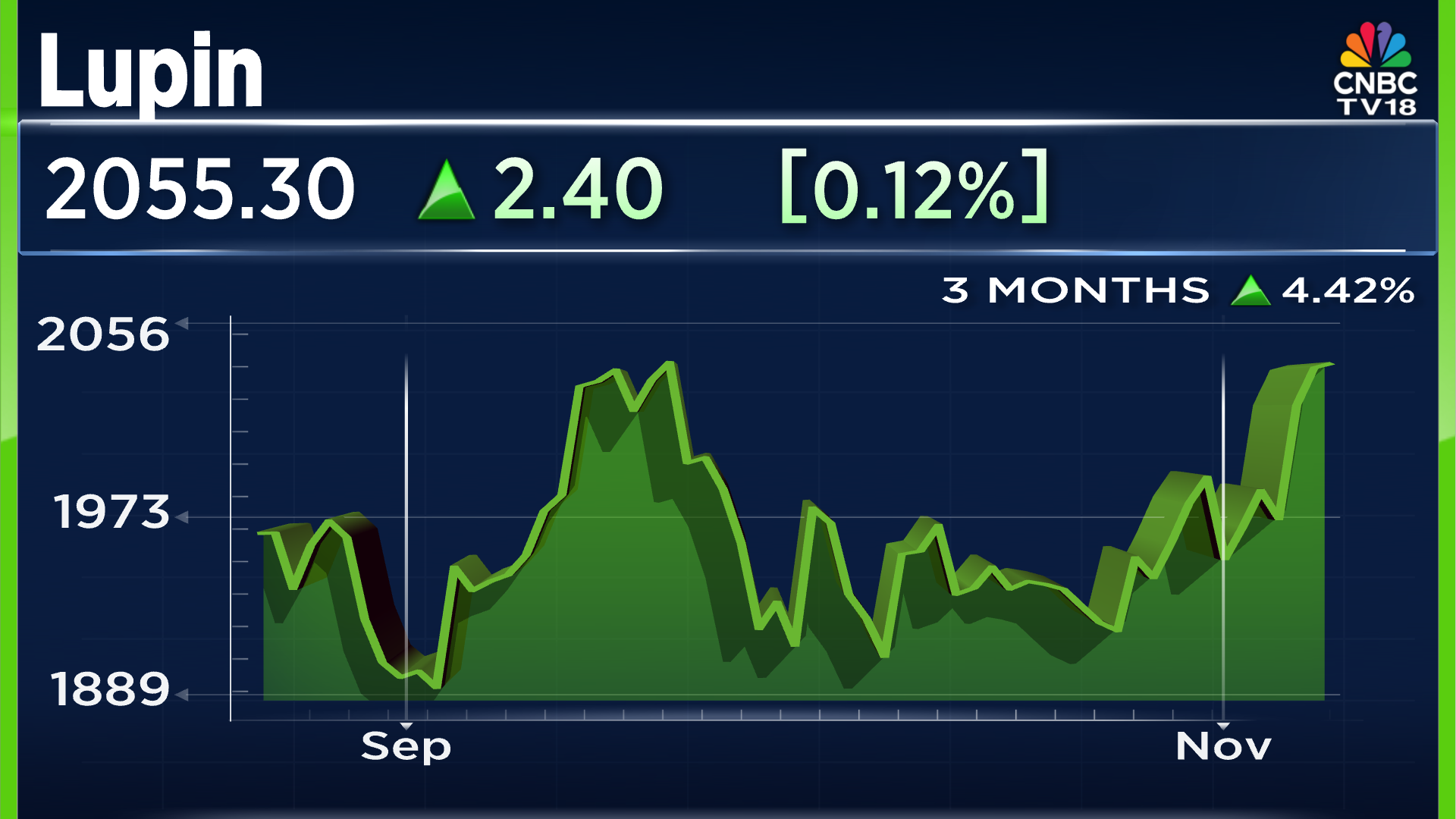
Moving to the company stock's recent performance, the company shares saw a minor rise on Friday's (November 14) session, with an uptick of 0.12% or ₹2.40. In the past 3 months, the company's shares have seen a rise of 4.42%, taking the overall value of the stock to ₹2,055.30 per share.
Also Read: Lupin Q2 Results | Net profit zooms 73% driven by higher revenue; exceeds poll estimates
In an exchange filing published on November 15, the pharma company said that the drug regulator concluded the inspection of its Nagpur Unit-1 Facility with "No Observations”.
This inspection from the USFDA was carried out as a part of a PreApproval Inspection at its Unit-1 oral solid dosage manufacturing facility.
This inspection closed with zero FDA 483 observations.
Here, the FDA's 483 observation is a notice issued by the US-based regulatory body's inspector to a pharma company after an inspection, listing any potential regulatory violations found at the manufacturing facility.
The inspection from officials lasted for 4 days; it was carried out from November 10 to November 14, 2025.
Talking about the 'successful outcome', Nilesh Gupta, Managing Director, Lupin, said, “The successful outcome of the US FDA inspection at our Nagpur Unit-1 facility exemplifies our commitment to uphold and maintain the highest standards of quality, compliance, and safety across our facilities. We remain dedicated to improving the lives of our patients globally.”
When we take a look at the company's recent performance, in its Q2FY26 results, the company reported a 73.34% year-on-year surge in net profit to ₹1,478 crore for Q2.
Revenue from operations grew 24.2% year-on-year to ₹7,047.5 crore. When we look at other crucial facets, the Earnings before interest, tax, depreciation, and amortisation (EBITDA) saw a rise of 74.7% to ₹2,341.7 crore, against ₹1,340.5 crore in the previous cycle.
Furthermore, when we gauge the profitability, the company's margin grew to 33.2%, a near 100 basis point rise from the previous year's 23.6%.

Moving to the company stock's recent performance, the company shares saw a minor rise on Friday's (November 14) session, with an uptick of 0.12% or ₹2.40. In the past 3 months, the company's shares have seen a rise of 4.42%, taking the overall value of the stock to ₹2,055.30 per share.
Also Read: Lupin Q2 Results | Net profit zooms 73% driven by higher revenue; exceeds poll estimates
/images/ppid_59c68470-image-176321254073021367.webp)







/images/ppid_a911dc6a-image-177106302538573741.webp)





