What is the story about?
The shares of Aurobindo Pharma will be in focus in today's session, as the USFDA issued a Form 483 with a total of nine observations post the inspection.
The Hyderabad-based company, in an exchange filing, said the FDA inspected Aurobindo Pharma’s Unit-VII, an oral solid dosage manufacturing unit situated at Special Economic Zone (Pharma), TSIIC, Green Industrial Park,
The US regulatory body conducted these inspections from January 28 to February 10, 2026.
At the end of this inspection, the US FDA issued a ‘Form 483’ with a total of 9 observations.
Here, the Form 483 could document potential violations regarding facility, equipment, processes, controls, products, employee practices, or records.
Previously, on February 9, the company had reported a the USFDA conducted an inspection at the company's Unit III manufacturing facility.
Also Read: Aurobindo Pharma shares fall after USFDA issues 11 observations at Unit III
The company also reported its Q3 results on Monday, February 9, wherein it reported its third-quarter results, posting a net profit of ₹909.8 crore. The bottom-line figures rose 7.5% year-on-year from ₹846 crore, inclusive of a one-time cost of ₹65 crore due to a change in the labour code.
In addition, the top-line figures also grew for the quarter, growing 8.4% year-on-year to ₹8,646 crore. This was higher than the amount compared with last year's ₹7,979 crore. This rise was supported by growth in Europe and the ARV (Antiretroviral drugs) segment.
In its latest rating, CLSA maintains an 'Outperform' rating. In addition, it raised the target price on the stock to ₹1370 from its previous ₹1340 earlier
Furthermore, DAM Capital issued a 'BUY' call with a target price of ₹1513.
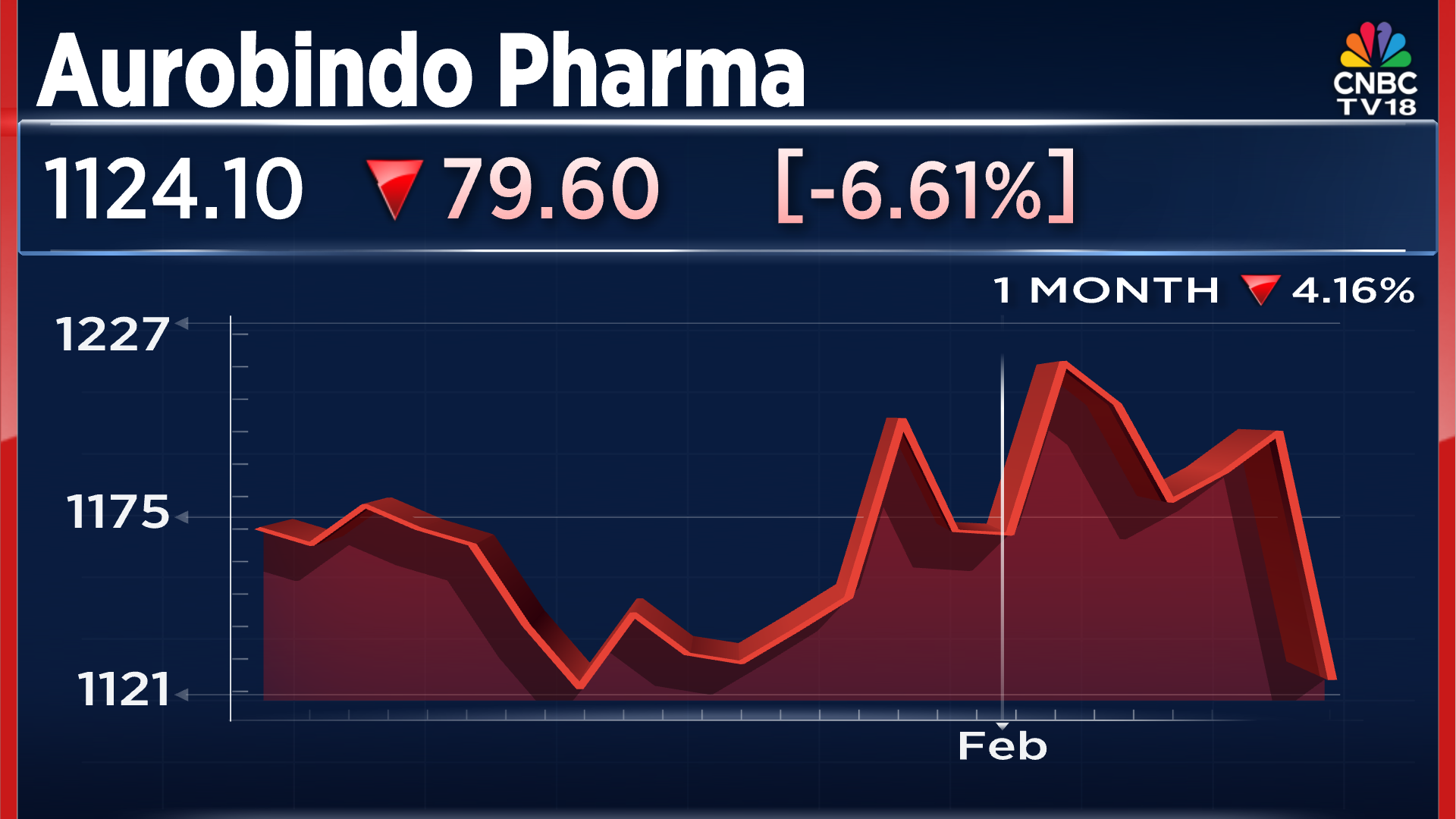
When we take a quick look at the stock performance, the company's shares declined by over 6% in yesterday's trading session. In the past month of trade, the company's stock price has fallen by over 4%. The current stock price stands at ₹1,124 per share.
Also Read: Aurobindo Pharma Q3 Results: Net profit up 8% on Europe, ARV growth despite labour code cost
The Hyderabad-based company, in an exchange filing, said the FDA inspected Aurobindo Pharma’s Unit-VII, an oral solid dosage manufacturing unit situated at Special Economic Zone (Pharma), TSIIC, Green Industrial Park,
The US regulatory body conducted these inspections from January 28 to February 10, 2026.
At the end of this inspection, the US FDA issued a ‘Form 483’ with a total of 9 observations.
Here, the Form 483 could document potential violations regarding facility, equipment, processes, controls, products, employee practices, or records.
Previously, on February 9, the company had reported a the USFDA conducted an inspection at the company's Unit III manufacturing facility.
Also Read: Aurobindo Pharma shares fall after USFDA issues 11 observations at Unit III
The company also reported its Q3 results on Monday, February 9, wherein it reported its third-quarter results, posting a net profit of ₹909.8 crore. The bottom-line figures rose 7.5% year-on-year from ₹846 crore, inclusive of a one-time cost of ₹65 crore due to a change in the labour code.
In addition, the top-line figures also grew for the quarter, growing 8.4% year-on-year to ₹8,646 crore. This was higher than the amount compared with last year's ₹7,979 crore. This rise was supported by growth in Europe and the ARV (Antiretroviral drugs) segment.
In its latest rating, CLSA maintains an 'Outperform' rating. In addition, it raised the target price on the stock to ₹1370 from its previous ₹1340 earlier
Furthermore, DAM Capital issued a 'BUY' call with a target price of ₹1513.

When we take a quick look at the stock performance, the company's shares declined by over 6% in yesterday's trading session. In the past month of trade, the company's stock price has fallen by over 4%. The current stock price stands at ₹1,124 per share.
Also Read: Aurobindo Pharma Q3 Results: Net profit up 8% on Europe, ARV growth despite labour code cost
/images/ppid_59c68470-image-177078007003694796.webp)

/images/ppid_59c68470-image-177077508321758455.webp)
/images/ppid_a911dc6a-image-177077322976413782.webp)
/images/ppid_59c68470-image-177077258165510745.webp)
/images/ppid_a911dc6a-image-177077884378925186.webp)

/images/ppid_59c68470-image-177077760046699504.webp)
/images/ppid_59c68470-image-177077752738996998.webp)
/images/ppid_59c68470-image-177077764100798849.webp)
/images/ppid_59c68470-image-177077755993327863.webp)
/images/ppid_59c68470-image-177077759049498278.webp)
/images/ppid_59c68470-image-177077754454222790.webp)
/images/ppid_59c68470-image-177077753316323586.webp)
/images/ppid_a911dc6a-image-177077683848562681.webp)
/images/ppid_a911dc6a-image-177077603394625504.webp)