Sun Pharma Recall
As per its latest Enforcement Report, the US arm of Sun Pharma is in the process of recalling over 26,000 bottles of a generic medication that is used in the treatment of dandruff and skin conditions with inflammation and itching.
The entity initiated a Class III nationwide recall in the United States to recall on December 30, 2025.
The Princeton-based Sun Pharmaceutical Industries Inc., the US arm of the Mumbai-based company, is recalling 24,624 bottles of Fluocinolone Acetonide Solution Topical Solution for "Failed Impurities/Degradation Specifications".
In addition to inflammation and itching drugs, the USFDA added that the company is also recalling batches of a medication to treat acne vulgaris.
Sun Pharmaceutical Industries Inc initiated the Class III recall of Clindamycin Phosphate USP on November 26, 2025, due to "Failed Impurities/Degradation: Out of Specification results for Total Impurities and for Assay", it added.
Cipla Recall
When it comes to Cipla, a Nifty 50 stock, the USFDA announced the action from this company in a separate disclosure.
The USFDA said that Cipla is recalling 15,221 syringes in the American market.
According to the US drug regulator, the Warren-based arm of the Mumbai-based Cipla is recalling the Lanreotide Injection, 120 mg/0.5 mL, 0.5 mL per pre-filled syringe. This recall has been initiated due to the "presence of particulate matter".
The company initiated the Class II nationwide recall on January 2 this year.
What Are Class II And Class III Recalls?
A Class II recall is initiated when the use of, or exposure to, a product in violation of norms could lead to temporary health consequences, or when the possibility of a serious adverse health outcome is limited.
On the other hand, a Class III recall is initiated in a "situation in which use of, or exposure to, a violative product is not likely to cause adverse health consequences".
As per the USFDA, a Class III recall is initiated in a "situation in which use of, or exposure to, a violative product is not likely to cause adverse health consequences".

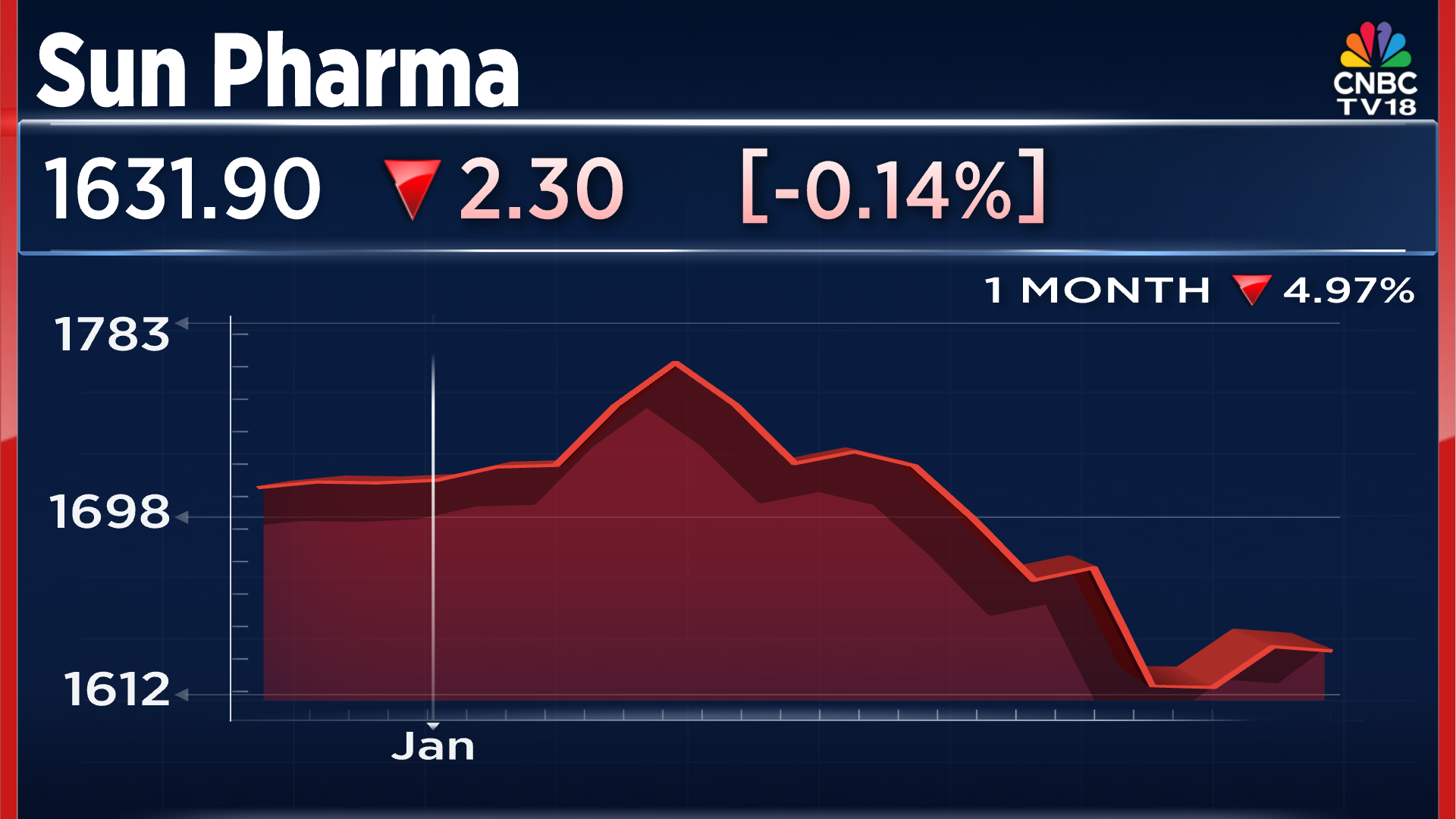
When it comes to Cipla, the company's shares have declined close to 12% in the past month of trade, taking the share price to ₹1,315 per piece. The shares of Sun Pharma have declined close to 5% in the past month of trade, falling to ₹1,631 per share.
Also Read: What’s driving silver’s sharp rally
/images/ppid_59c68470-image-176948756719653106.webp)




/images/ppid_a911dc6a-image-177084257810510483.webp)

/images/ppid_a911dc6a-image-177084253417916956.webp)

/images/ppid_59c68470-image-177084004043125742.webp)
/images/ppid_a911dc6a-image-177083903258342419.webp)


