What is the story about?
The shares of Indian pharmaceutical majors Sun Pharma and Cipla will be in focus today. According to the US Food and Drug Administration, both pharma companies are recalling products in the US due to manufacturing-related issues.
Sun Pharma Recall
As per its latest Enforcement Report, the US arm of Sun Pharma is in the process of recalling over 26,000 bottles of a generic medication that is used in the treatment of dandruff and skin conditions with inflammation and itching.
The entity initiated a Class III nationwide recall in the United States to recall on December 30, 2025.
The Princeton-based Sun Pharmaceutical Industries Inc., the US arm of the Mumbai-based company, is recalling 24,624 bottles of Fluocinolone Acetonide Solution Topical Solution for "Failed Impurities/Degradation Specifications".
In addition to inflammation and itching drugs, the USFDA added that the company is also recalling batches of a medication to treat acne vulgaris.
Sun Pharmaceutical Industries Inc initiated the Class III recall of Clindamycin Phosphate USP on November 26, 2025, due to "Failed Impurities/Degradation: Out of Specification results for Total Impurities and for Assay", it added.
Cipla Recall
When it comes to Cipla, a Nifty 50 stock, the USFDA announced the action from this company in a separate disclosure.
The USFDA said that Cipla is recalling 15,221 syringes in the American market.
According to the US drug regulator, the Warren-based arm of the Mumbai-based Cipla is recalling the Lanreotide Injection, 120 mg/0.5 mL, 0.5 mL per pre-filled syringe. This recall has been initiated due to the "presence of particulate matter".
The company initiated the Class II nationwide recall on January 2 this year.
What Are Class II And Class III Recalls?
A Class II recall is initiated when the use of, or exposure to, a product in violation of norms could lead to temporary health consequences, or when the possibility of a serious adverse health outcome is limited.
On the other hand, a Class III recall is initiated in a "situation in which use of, or exposure to, a violative product is not likely to cause adverse health consequences".
As per the USFDA, a Class III recall is initiated in a "situation in which use of, or exposure to, a violative product is not likely to cause adverse health consequences".

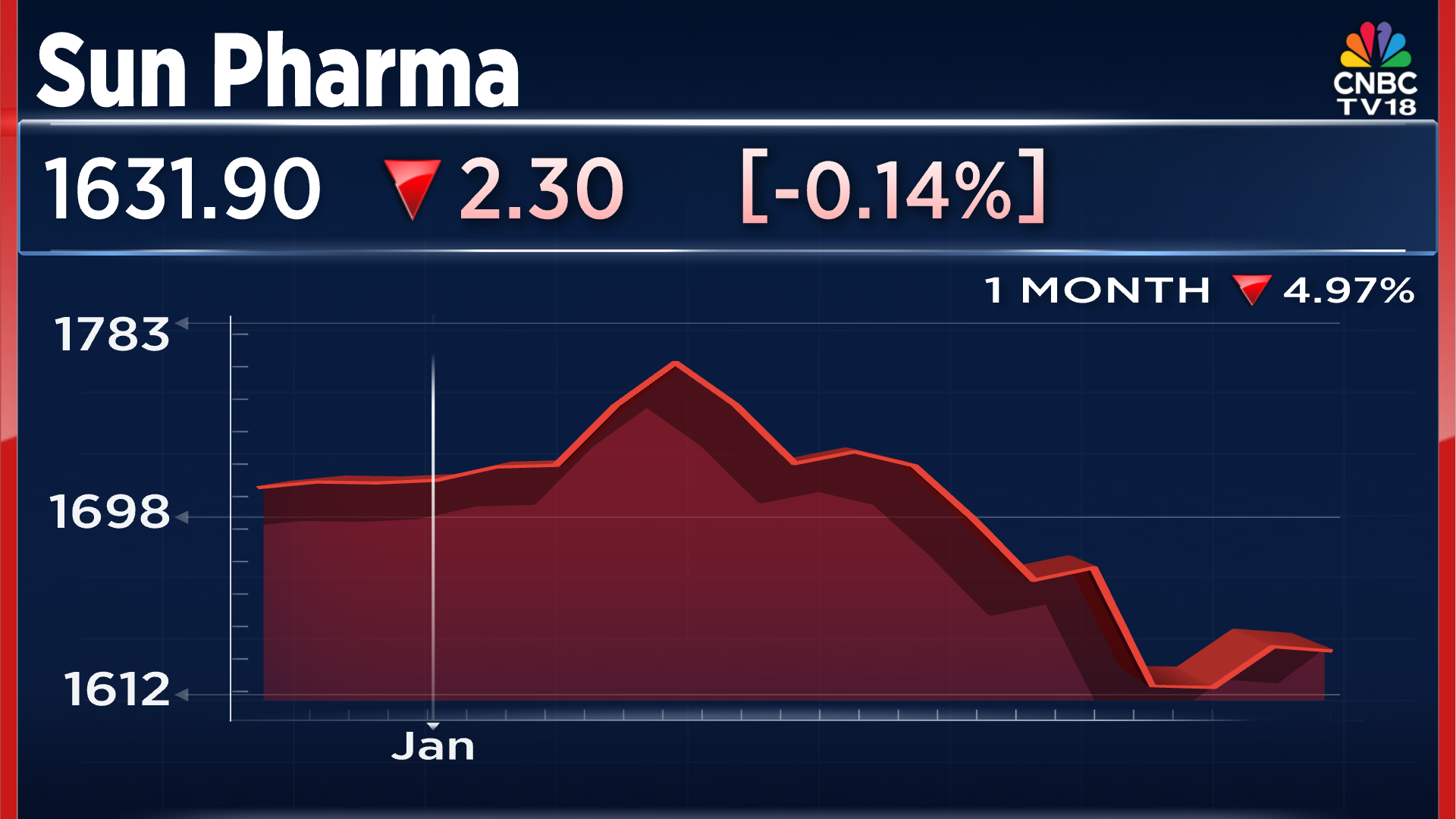
When it comes to Cipla, the company's shares have declined close to 12% in the past month of trade, taking the share price to ₹1,315 per piece. The shares of Sun Pharma have declined close to 5% in the past month of trade, falling to ₹1,631 per share.
Also Read: What’s driving silver’s sharp rally
Sun Pharma Recall
As per its latest Enforcement Report, the US arm of Sun Pharma is in the process of recalling over 26,000 bottles of a generic medication that is used in the treatment of dandruff and skin conditions with inflammation and itching.
The entity initiated a Class III nationwide recall in the United States to recall on December 30, 2025.
The Princeton-based Sun Pharmaceutical Industries Inc., the US arm of the Mumbai-based company, is recalling 24,624 bottles of Fluocinolone Acetonide Solution Topical Solution for "Failed Impurities/Degradation Specifications".
In addition to inflammation and itching drugs, the USFDA added that the company is also recalling batches of a medication to treat acne vulgaris.
Sun Pharmaceutical Industries Inc initiated the Class III recall of Clindamycin Phosphate USP on November 26, 2025, due to "Failed Impurities/Degradation: Out of Specification results for Total Impurities and for Assay", it added.
Cipla Recall
When it comes to Cipla, a Nifty 50 stock, the USFDA announced the action from this company in a separate disclosure.
The USFDA said that Cipla is recalling 15,221 syringes in the American market.
According to the US drug regulator, the Warren-based arm of the Mumbai-based Cipla is recalling the Lanreotide Injection, 120 mg/0.5 mL, 0.5 mL per pre-filled syringe. This recall has been initiated due to the "presence of particulate matter".
The company initiated the Class II nationwide recall on January 2 this year.
What Are Class II And Class III Recalls?
A Class II recall is initiated when the use of, or exposure to, a product in violation of norms could lead to temporary health consequences, or when the possibility of a serious adverse health outcome is limited.
On the other hand, a Class III recall is initiated in a "situation in which use of, or exposure to, a violative product is not likely to cause adverse health consequences".
As per the USFDA, a Class III recall is initiated in a "situation in which use of, or exposure to, a violative product is not likely to cause adverse health consequences".


When it comes to Cipla, the company's shares have declined close to 12% in the past month of trade, taking the share price to ₹1,315 per piece. The shares of Sun Pharma have declined close to 5% in the past month of trade, falling to ₹1,631 per share.
Also Read: What’s driving silver’s sharp rally
/images/ppid_59c68470-image-176948756719653106.webp)




/images/ppid_a911dc6a-image-177088864928744173.webp)



/images/ppid_a911dc6a-image-177088805007765911.webp)





/images/ppid_a911dc6a-image-177088803864211353.webp)
/images/ppid_59c68470-image-177088756453137106.webp)
