What is the story about?
What's Happening?
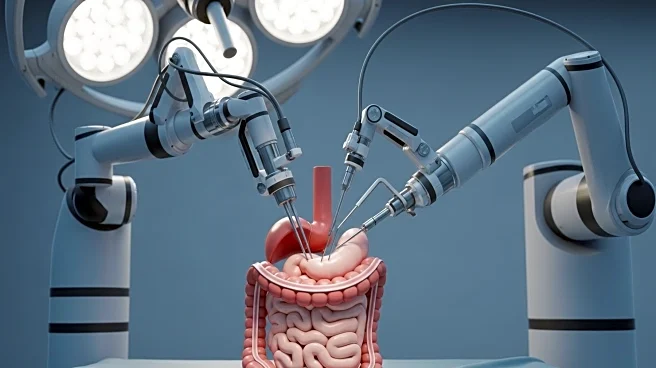
EndoQuest Robotics Inc. has announced the successful completion of a robotic endoscopic submucosal dissection (ESD) procedure at the Mayo Clinic. This procedure is part of the Prospective Assessment of a Robotic Assisted Device in Gastrointestinal Medicine (PARADIGM) Trial, which aims to evaluate the company's Endoluminal Surgical System for lower gastrointestinal tract procedures. Dr. Norio Fukami, a professor of medicine and director of Therapeutic Endoscopy at Mayo Clinic, performed the procedure to remove a complex colorectal lesion. The trial is conducted under a U.S. Food and Drug Administration (FDA) Investigational Device Exemption (IDE) pivotal trial. The ESD procedure, traditionally challenging, was made more straightforward with the use of the Endoluminal Surgical System, which provides dynamic traction and counter-traction capabilities.
Why It's Important?
The successful completion of this procedure marks a significant advancement in gastrointestinal medicine, potentially reducing the need for invasive surgeries like colectomies. The Endoluminal Surgical System is designed to simplify complex endoluminal procedures, which are typically difficult due to the limitations of standard endoscopic tools. This technology could lead to a shift towards more organ-sparing treatments, reducing the burden on patients and healthcare providers. The growing interest in advanced endoscopy is evidenced by a more than 50% increase in fellowship programs over the past decade, indicating a strong demand for innovative solutions like those offered by EndoQuest.
What's Next?
The PARADIGM Trial will continue to enroll 50 subjects across five major U.S. healthcare institutions, including Brigham and Women’s Hospital and Cleveland Clinic. Upon completion, EndoQuest plans to submit a De Novo request for FDA authorization to market the Endoluminal Surgical System in the U.S. The company recently secured Series D-2 funding to support the advancement of its surgical platform and the ongoing clinical trial. The system is currently investigational and not yet available for commercial sale in the U.S.
Beyond the Headlines
The introduction of robotic-assisted endoluminal procedures could have long-term implications for the field of therapeutic endoscopy, potentially setting new standards for minimally invasive treatments. This could lead to broader acceptance and integration of robotic systems in gastrointestinal procedures, fostering further innovation and development in medical robotics.