What's Happening?
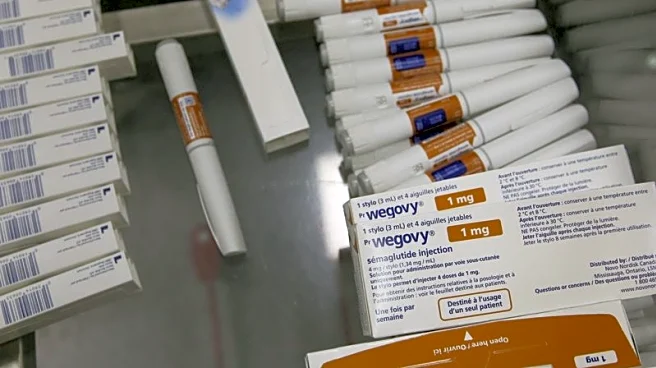
Hengrui Medicine has announced successful Phase III trial results for its obesity drug, HRS9531, a dual GLP-1/GIP receptor agonist. The drug demonstrated significant weight loss efficacy, with participants losing an average of 17.7% of their weight after 48 weeks of treatment. This positions HRS9531 as a strong competitor against Eli Lilly's Zepbound and Novo Nordisk's Wegovy. Hengrui's strategic partnerships and financial backing, including a $200 million deal with Merck, support its global expansion plans and trial execution.
Why It's Important?
Hengrui Medicine's breakthrough in obesity therapeutics could disrupt the global GLP-1 market, which is projected to grow significantly. The drug's competitive pricing and efficacy may attract both payers and patients, especially in cost-sensitive regions. Hengrui's strategic partnerships and focus on global trials highlight its potential to challenge established players like Eli Lilly and Novo Nordisk, potentially reshaping market dynamics and accessibility to obesity treatments.
What's Next?
Hengrui plans to file for regulatory approval in China by late 2025, while its U.S. partner, Kailera Therapeutics, advances global trials targeting higher BMI populations. The company aims to leverage accelerated approval pathways in China and address unmet needs in international markets. Hengrui's broader pipeline, including oral GLP-1 agonists, may further enhance its market position.