What is the story about?
What's Happening?
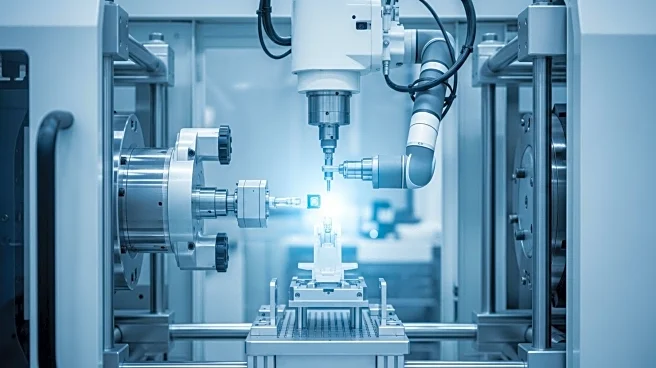
Kistler is set to showcase its AI-supported automated quality control system for injection molding in the medical technology sector at the K 2025 event in Düsseldorf. This innovation marks a shift from traditional random sampling to a comprehensive 100 percent inspection of each part produced. The system utilizes AI to calculate key product characteristics based on data such as cavity pressure, directly sourced from the injection molding tool. This approach is particularly crucial for the production of medical devices like auto-injectors, which are in high demand due to the increasing prevalence of diseases such as diabetes and Parkinson's. The technology aims to ensure reliability and safety in medical devices, preventing life-threatening malfunctions and providing manufacturers with proof of exhaustive error prevention measures.
Why It's Important?
The introduction of AI-supported quality control in injection molding is significant for the medical device industry, which faces growing demand and the need for rapid production ramp-ups. Ensuring the reliability of devices like insulin pens is critical, as any malfunction can have severe consequences for users. This technology offers a more thorough quality assurance method compared to traditional statistical process control, enhancing safety and compliance with FDA and MDR standards. Manufacturers stand to benefit from reduced risk of production errors and improved product safety, which can lead to increased trust and market competitiveness.
What's Next?
Kistler will demonstrate its complete measurement chain for automated quality control at the K 2025 event, including sensors, process monitoring, and data documentation systems. The company aims to support medical technology manufacturers in implementing this advanced quality assurance method efficiently. As more manufacturers adopt this technology, it could lead to widespread improvements in production processes and product safety across the industry.
Beyond the Headlines
The shift to AI-supported quality control in medical device manufacturing could have broader implications for the industry, including potential changes in regulatory standards and practices. As manufacturers increasingly rely on AI and data-driven processes, there may be ethical considerations regarding data privacy and the transparency of AI algorithms used in quality assurance.