What's Happening?
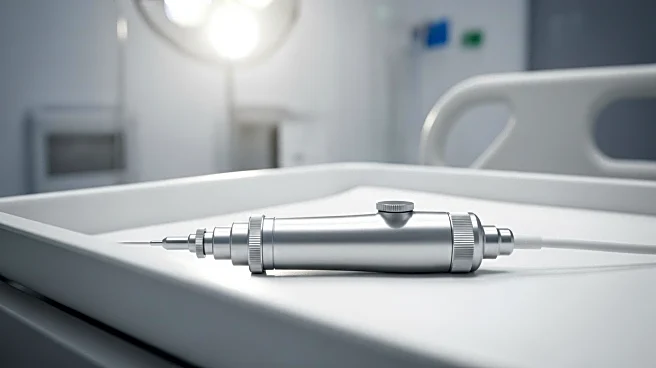
Olympus has initiated a recall of certain lots of its ViziShot 2 FLEX needles used in lung biopsy procedures after reports of patient injuries and one death. The recall was prompted by concerns that components of the needles may detach during procedures, posing a risk to patients. The ViziShot 2 FLEX needles are utilized in minimally invasive procedures to diagnose or stage lung cancer, guided by ultrasound to collect tissue samples. Olympus has faced quality concerns in recent years, with the FDA issuing multiple warning letters related to its endoscopes and blocking some devices. The company has pledged a prompt response following the FDA's import block.
Why It's Important?
The recall of Olympus' ViziShot 2 FLEX needles highlights ongoing safety and quality concerns within the medical device industry. This development is significant as it underscores the critical need for stringent quality control measures to ensure patient safety. The recall may impact Olympus' reputation and financial performance, as well as influence regulatory scrutiny on similar medical devices. Healthcare providers and patients relying on these devices for diagnostic procedures may face disruptions, potentially affecting treatment timelines and outcomes.
What's Next?
Olympus is expected to address the quality issues and work closely with the FDA to resolve the import block and ensure compliance with safety standards. The company may need to implement additional quality control measures and conduct thorough investigations to prevent future incidents. Healthcare providers may seek alternative diagnostic tools, and patients may experience delays in procedures. Regulatory bodies may increase scrutiny on medical device manufacturers to prevent similar occurrences.