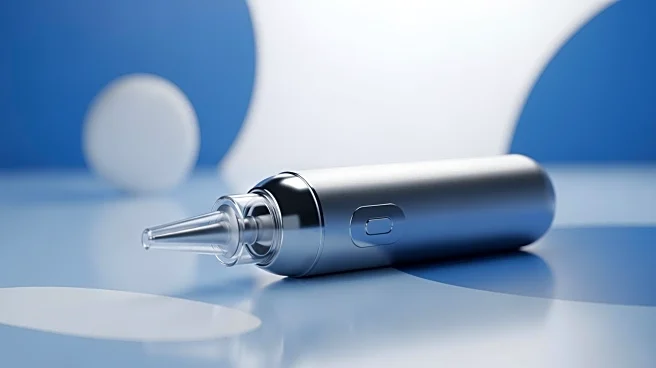
What's Happening?
Aptar Group, Inc. has announced that its Bidose Nasal Spray System is being used to deliver CARDAMYST (etripamil), the first FDA-approved self-administered nasal spray for paroxysmal supraventricular tachycardia
(PSVT). Developed in collaboration with Milestone Pharmaceuticals, this nasal spray offers a novel treatment for PSVT, a condition characterized by rapid heartbeats. The Bidose system is designed for ease of use, allowing patients to carry and administer the treatment conveniently. This approval highlights Aptar's expanding role in drug delivery solutions, particularly in the area of nasal drug delivery.
Why It's Important?
The introduction of CARDAMYST as a self-administered treatment for PSVT represents a significant advancement in the management of this condition, which affects approximately two million people in the U.S. annually. By enabling patients to manage their symptoms outside of a clinical setting, the nasal spray could reduce emergency department visits and hospitalizations, leading to cost savings for the healthcare system. Aptar's involvement in this project underscores the growing demand for innovative drug delivery systems that enhance patient autonomy and treatment accessibility.