What's Happening?
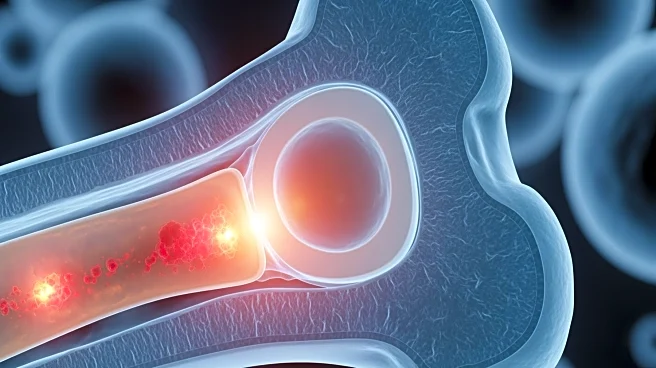
A study has revealed that inflammation in the bone marrow microenvironment plays a crucial role in the early stages of disease development, particularly in conditions like clonal hematopoiesis of indeterminate potential (CHIP) and myelodysplastic syndrome
(MDS). Researchers found that inflammatory stromal cells replace normal mesenchymal stromal cells, disrupting stem-cell function and contributing to disease progression. This inflammatory activity creates a feedback loop that maintains chronic inflammation, affecting blood formation and vascular changes in the marrow. The study suggests that anti-inflammatory drugs or therapies targeting interferon signaling could help preserve marrow function and prevent the transition from CHIP to MDS or acute myeloid leukemia (AML).
Why It's Important?
Understanding the role of inflammation in the bone marrow microenvironment offers new opportunities for early treatment and prevention of blood diseases. By focusing on the ecosystem that supports mutated stem cells, rather than the mutated cells alone, researchers can develop preventive therapies that intercept disease progression before leukemia develops. This approach could significantly reduce the risk of blood cancers and improve patient outcomes. Additionally, the findings contribute to a broader understanding of 'inflammaging', the chronic inflammation associated with many age-related conditions, including cancer and cardiovascular disease. This research highlights the potential for targeted therapies to address systemic inflammatory aging.
What's Next?
Further studies are needed to explore the long-term effects of inflammation on the bone marrow microenvironment and its role in disease progression. Researchers are investigating how the bone marrow niche retains a 'memory' of disease, which could influence its response to new, healthy stem cells. This has implications for therapies that replace malignant cells but leave the bone marrow niche intact, such as blood stem cell transplantation. The study also opens avenues for exploring inflammatory remodeling in other myeloid malignancies and advanced leukemia, potentially leading to new therapeutic strategies.