What's Happening?
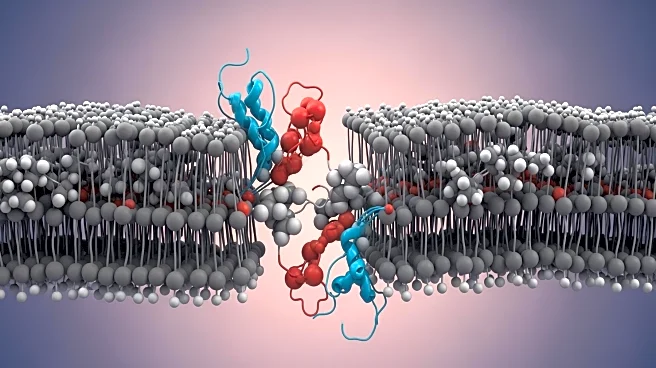
A recent study published in Nature investigates the mechanisms of cholesterol attraction in transmembrane helices. The research utilizes bioinformatic approaches, molecular modeling, and simulations to
identify cholesterol recognition motifs in various membrane protein families. The study highlights the role of phenylalanine within the CARC motif in enhancing protein-protein interactions within cholesterol-enriched lipid membranes. The findings suggest that cholesterol recognition is influenced by thermodynamic forces dictated by amino acid composition and structural features of transmembrane helices, rather than solely by molecular shape compatibility.
Why It's Important?
Understanding cholesterol attraction mechanisms in membrane proteins is crucial for developing therapeutic strategies targeting cholesterol-related diseases. The study's insights into the structural determinants of cholesterol preference could inform drug design and improve treatments for conditions like cardiovascular disease and metabolic disorders. By elucidating the role of specific amino acids and motifs in cholesterol binding, researchers can better predict protein behavior in cholesterol-rich environments, potentially leading to more effective interventions.
What's Next?
Future research may focus on applying these findings to real-world applications, such as drug development and disease treatment. Researchers could explore the therapeutic potential of manipulating cholesterol attraction in membrane proteins to address cholesterol-related health issues. Additionally, further studies might investigate the broader implications of these mechanisms in cellular processes and their impact on human health.
Beyond the Headlines
The study raises questions about the evolutionary conservation of cholesterol attraction mechanisms and their role in cellular function. It suggests that cholesterol binding may be more complex than previously thought, involving a balance of structural and thermodynamic factors. This could lead to a reevaluation of existing models of cholesterol interaction in biological membranes.