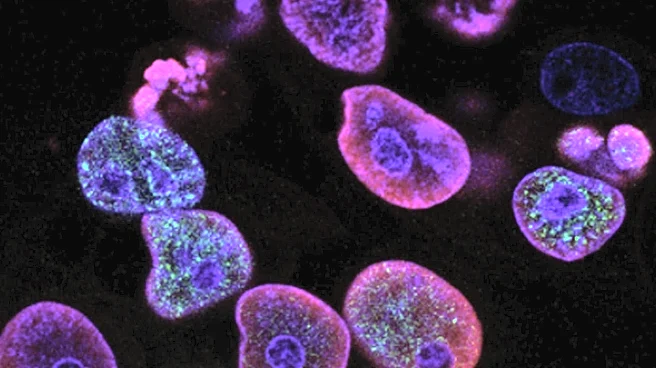
What's Happening?
Recent research has highlighted a novel approach to combat drug tolerance in Mycobacterium tuberculosis (Mtb) by reprogramming the bioenergetics of macrophages. The study focused on the transcriptional profiling of macrophage sub-populations infected
with Mtb, revealing factors that promote redox diversity and drug tolerance. By manipulating the energy metabolism of macrophages, researchers found that drug tolerance in Mtb could be reduced. The study utilized primary mouse bone marrow-derived macrophages infected with Mtb strains carrying a redox biosensor, which allowed for the identification of macrophage subsets enriched with either drug-tolerant or drug-sensitive phenotypes. The findings suggest that the metabolic state of macrophages, particularly the balance between oxidative phosphorylation and glycolysis, plays a crucial role in the redox-dependent drug tolerance of Mtb.
Why It's Important?
This research is significant as it offers a potential new strategy for enhancing the effectiveness of tuberculosis treatments. By targeting the bioenergetic pathways within macrophages, it may be possible to reduce the drug tolerance of Mtb, thereby improving the efficacy of existing antibiotics. This approach could lead to shorter treatment durations and better outcomes for patients suffering from tuberculosis, a disease that remains a major global health challenge. The study also underscores the importance of understanding host-pathogen interactions and the role of host metabolism in infectious disease management.
What's Next?
Further research is needed to explore the clinical applications of these findings. Potential next steps include testing the bioenergetic reprogramming approach in human macrophages and evaluating its effectiveness in combination with current tuberculosis treatments. Additionally, the development of drugs that can safely and effectively modulate macrophage metabolism without adverse effects will be crucial. Researchers may also investigate the broader implications of this approach for other infectious diseases where drug tolerance is a challenge.
Beyond the Headlines
The study raises ethical and scientific questions about the manipulation of host metabolism as a therapeutic strategy. Long-term effects and safety of altering macrophage bioenergetics need thorough investigation. Moreover, this approach could pave the way for new treatments targeting host-pathogen interactions, potentially revolutionizing the field of infectious disease therapy.