What's Happening?
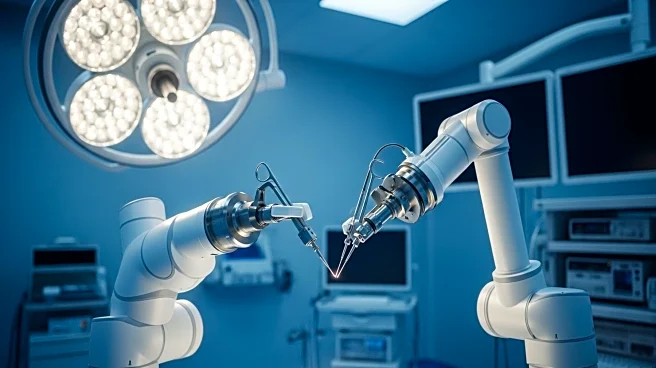
Medical Microinstruments Inc. (MMI) has successfully completed the first cases in a neurosurgical trial using its Symani Surgical System. The trial, sponsored by the Jacobs Institute, took place at Buffalo General Medical Center/Gates Vascular Institute. The Symani system, known for its precision in delicate maneuvers, was used in procedures aimed at restoring blood supply to the brain in patients with Moyamoya Disease. The system combines micro-instruments with tremor-reducing and motion-scaling technologies, offering enhanced precision for complex surgeries. The FDA has authorized the system for use in the U.S., although it has not been cleared for neurosurgical applications.
Why It's Important?
The successful application of the Symani Surgical System in neurosurgery represents a significant advancement in robotic-assisted surgery. This development could potentially transform the treatment of neurovascular diseases, offering new opportunities for precision in surgeries that require delicate handling. The system's ability to perform minute surgical moves on the pulsating brain may lead to improved outcomes for patients suffering from conditions like stroke, seizures, and paralysis. As robotic technology continues to evolve, it may redefine surgical practices, enhancing the capabilities of surgeons and improving patient care.
What's Next?
The ongoing feasibility study will continue to assess the safety and effectiveness of the Symani system in neurosurgery. Dr. Adnan Siddiqui, who performed the initial procedures, will present the findings at the Congress of Neurological Surgeons Annual Meeting. The study's results could pave the way for broader adoption of robotic systems in neurosurgery, potentially influencing future regulatory approvals and clinical practices. As the technology gains recognition, it may attract further investment and research, driving innovation in the field of robotic surgery.