What is the story about?
What's Happening?
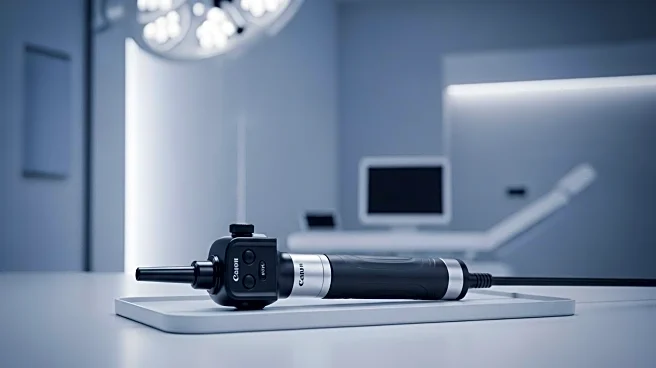
Zenflow, Inc., a medical device company based in South San Francisco, has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its Zenflow Spring® Scope & Camera Control Unit (CCU). This device is the first of its kind, offering a single-use cystoscope with a 12 French working channel, which is significantly larger than existing models. This advancement allows urologists to perform procedures with enhanced visualization while maintaining patient comfort. Flexible cystoscopy is a common procedure used to diagnose and treat lower urinary tract conditions, with approximately 1.2 million cystoscopies performed annually in the United States. The Zenflow Spring Scope aims to improve the diagnostic and therapeutic capabilities available to urologists in office settings.
Why It's Important?
The FDA clearance of Zenflow's Spring Scope represents a significant advancement in urologic care, particularly for patients with benign prostatic hyperplasia (BPH). The larger working channel and improved imaging capabilities of the device promise to enhance the accuracy and comfort of cystoscopic procedures. This innovation is expected to benefit both patients and healthcare providers by streamlining office-based diagnostics and treatments. The development aligns with Zenflow's commitment to patient-focused innovation, potentially setting a new standard in the field of urology and influencing future medical device designs.
What's Next?
With the FDA clearance secured, Zenflow is poised to introduce its Spring Scope to the market, potentially transforming urologic care practices. The company is also developing the Zenflow Spring Implant and Delivery System, which is currently investigational and not yet approved for commercial sale. As Zenflow continues to innovate, the medical community may anticipate further advancements in BPH therapy and other urologic treatments. Stakeholders, including healthcare providers and patients, will likely monitor the rollout of these technologies and their impact on clinical practices.
Beyond the Headlines
The introduction of single-use medical devices like the Zenflow Spring Scope may have broader implications for healthcare economics and environmental sustainability. Single-use devices can reduce the risk of cross-contamination and infection, potentially lowering healthcare costs associated with hospital-acquired infections. However, they also raise questions about medical waste and environmental impact, prompting discussions on sustainable practices in medical device manufacturing and disposal.