What is the story about?
What's Happening?
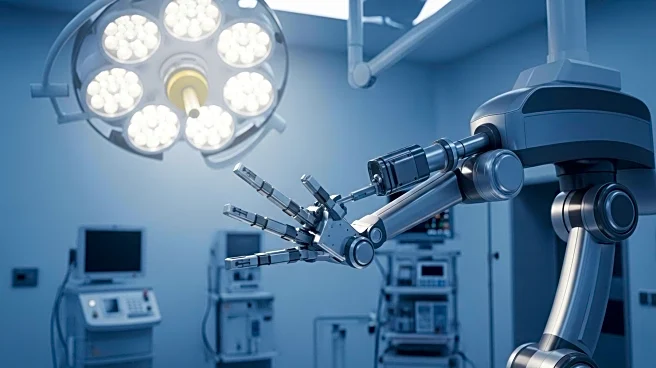
Microbot Medical has announced that its Liberty endovascular surgical robot has received 510(k) clearance from the U.S. Food and Drug Administration (FDA). This clearance marks Liberty as the first FDA-approved single-use, remotely operated robotic system for peripheral endovascular procedures. The Liberty system is designed to improve procedural precision, safety, and efficiency for interventional physicians and hospitals. It offers a compact, single-use solution that allows for controlled remote operation, which is a significant advancement over traditional manual techniques. The pivotal study for Liberty demonstrated 100% success in robotic navigation to target areas and reported zero device-related adverse events. Additionally, the study showed a 92% reduction in radiation exposure for physicians, highlighting the system's potential to enhance safety during procedures.
Why It's Important?
The FDA clearance of the Liberty surgical robot is a significant milestone for Microbot Medical and the field of endovascular robotics. This development is expected to expand access to advanced robotic technologies, addressing critical unmet needs in healthcare while supporting cost-effective solutions. The Liberty system's ability to reduce radiation exposure and improve procedural efficiency could lead to better patient outcomes and lower healthcare costs. As the system is positioned for commercialization in the U.S., it has the potential to penetrate the market of approximately 2.5 million annual peripheral vascular procedures, offering a safer and more efficient alternative to existing methods.
What's Next?
With FDA clearance secured, Microbot Medical is set to complete the final commercial activities necessary for market entry. The company plans to commence commercialization efforts and pursue entry into global markets. Additionally, Microbot Medical is exploring wider applications for the Liberty system, supported by a new patent covering a modular robotic surgical system. This patent could enable the adaptation of Liberty for a broader range of endovascular procedures, further expanding its market potential.