What's Happening?
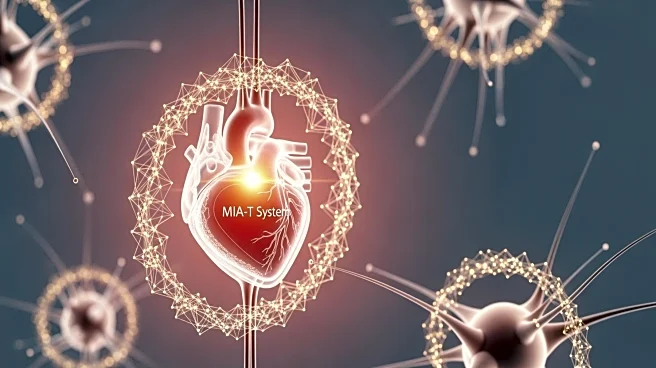
Micro Interventional Devices (MID), a company specializing in Transcatheter Tricuspid Valve Repair (TTVr), has received approval from the U.S. Food & Drug Administration (FDA) for its MIA-T Percutaneous Tricuspid Annuloplasty System. This approval allows
MID to commence the STTAR-US IDE pivotal trial at prominent U.S. hospitals. The trial will utilize MID's PolyCor™ anchors, designed for cardiac tissue, to treat functional tricuspid valve disease by reducing annular area and minimizing Tricuspid Regurgitation (TR). The trial is led by cardiologists Bassem Chehab, MD, and Saibal Kar, MD, who are recognized leaders in the structural heart field. The MIA-T system aims to address the unmet needs of 1.6 million TR patients in the U.S., many of whom are ineligible for surgical treatment.
Why It's Important?
The FDA approval of the MIA-T system is a significant development in the treatment of tricuspid valve disease, offering a minimally invasive option for patients who cannot undergo surgery. This advancement is crucial as it addresses a large, untreated patient population, potentially improving patient outcomes and expanding treatment options. The global market for Transcatheter Tricuspid Valve Repair is projected to exceed $3 billion annually by 2027, indicating substantial growth and adoption of minimally invasive technologies. MID's MIA-T system could become a key player in this emerging market, providing value to stakeholders and enhancing patient care.
What's Next?
With the FDA approval, MID will begin the STTAR-US pivotal trial, which is expected to provide critical data on the efficacy and safety of the MIA-T system. The trial's outcomes could influence future regulatory decisions and market adoption. As the trial progresses, MID may seek further approvals and partnerships to expand the use of its technology. The success of the trial could lead to increased interest from healthcare providers and investors, potentially accelerating the development and commercialization of similar technologies.
Beyond the Headlines
The approval of the MIA-T system highlights the growing trend towards minimally invasive medical technologies, which can reduce recovery times and improve patient experiences. This shift may encourage further innovation in the field of structural heart disease treatment, potentially leading to new solutions for other cardiac conditions. Additionally, the trial's success could prompt discussions on healthcare policy and funding for advanced medical technologies, influencing how such treatments are integrated into standard care practices.