What is the story about?
What's Happening?
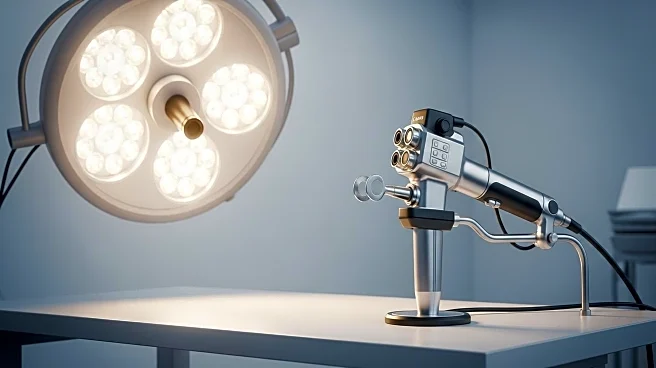
UroViu Corporation has received FDA 510(k) clearance for its UV5000 endoscope platform, a cordless, single-use device designed to improve endoscopic procedures. The UV5000 offers enhanced features such as a faster processor, longer battery life, and brighter HD visualization, compatible with UroViu's range of single-use endoscope cannulas and tools. The platform aims to simplify workflows and expand access to high-quality endoscopy by eliminating the need for reprocessing and capital equipment. UroViu's innovative design minimizes environmental impact and allows for greater procedural flexibility, turning any room into a procedure room. The UV5000 is now available in the U.S. market, with plans for a Wi-Fi enabled model, the UV5000W, pending FDA clearance.
Why It's Important?
The FDA clearance of UroViu's UV5000 platform marks a significant advancement in medical technology, offering healthcare providers a more efficient and environmentally friendly option for endoscopic procedures. By reducing the need for reprocessing and capital equipment, the UV5000 can lower operational costs and increase accessibility to high-quality medical care, particularly in settings with limited resources. This development is likely to benefit a wide range of healthcare facilities, including private practices, academic institutions, and government healthcare services, by enhancing procedural efficiency and patient care.
What's Next?
With the UV5000 now available in the U.S., UroViu is expected to focus on expanding its market presence and demonstrating the platform's benefits through hands-on demonstrations and partnerships with healthcare providers. The pending FDA clearance for the UV5000W model with Wi-Fi connectivity could further enhance the platform's capabilities, allowing for real-time data sharing and improved integration with external displays. As UroViu continues to innovate, the company may explore additional applications for its technology, potentially leading to new product developments and expanded market opportunities.