What's Happening?
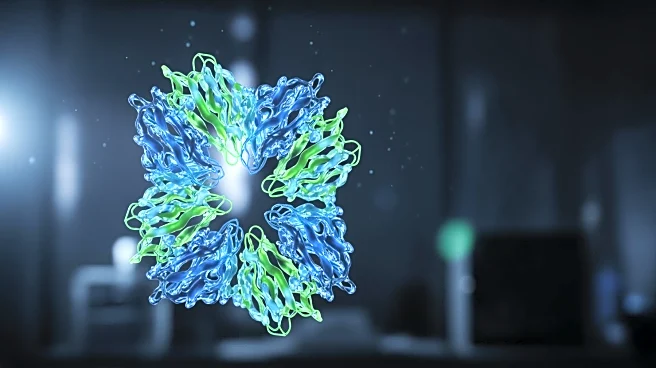
Researchers at the Salk Institute have successfully captured the structural changes of HIV-1 integrase, a key enzyme in the virus's replication cycle, using cryo-electron microscopy. This enzyme plays two distinct roles during the infection process: integrating
viral DNA into host chromatin during early infection stages and binding viral RNA during later stages to form the capsid core. The study, published in Nature Communications, reveals the molecular architectures of integrase in these roles for the first time, providing novel 3D models. The research highlights integrase's adaptability, which allows it to evade current drugs like Dolutegravir. The findings suggest that future therapeutics could target integrase's interaction with viral RNA, a newly discovered function, to combat drug resistance.
Why It's Important?
The discovery of integrase's structural states is significant for HIV research and drug development. Integrase is a critical target for HIV-1 drugs, but the virus's rapid evolution often leads to drug resistance. By understanding integrase's interaction with RNA, researchers can design new drugs that target this function, potentially overcoming resistance issues. This research provides a blueprint for developing more effective HIV therapeutics, which could significantly impact public health by improving treatment options for millions affected by HIV. The adaptability of integrase underscores the need for innovative approaches in drug design, emphasizing the importance of structural biology in addressing complex viral mechanisms.
What's Next?
The Salk Institute team plans to conduct follow-up studies to confirm their findings on integrase's interaction with RNA. These studies will further explore the protein's architecture and its implications for drug development. Researchers aim to leverage the new structural insights to design drugs that disrupt HIV replication more effectively. The ongoing research could lead to breakthroughs in HIV treatment, offering hope for more durable and less resistance-prone therapies. As the scientific community continues to investigate integrase's roles, collaboration with pharmaceutical companies may accelerate the development of novel drugs targeting these newly discovered functions.
Beyond the Headlines
The study of HIV-1 integrase's structural states not only advances drug development but also enhances our understanding of viral replication mechanisms. The adaptability of integrase highlights the complexity of HIV as a pathogen, necessitating a multifaceted approach to treatment. This research may inspire similar studies on other viral proteins, broadening the scope of antiviral drug development. Additionally, the use of cryo-electron microscopy in this research exemplifies the power of advanced imaging techniques in uncovering molecular details, potentially influencing future studies in virology and structural biology.