What's Happening?
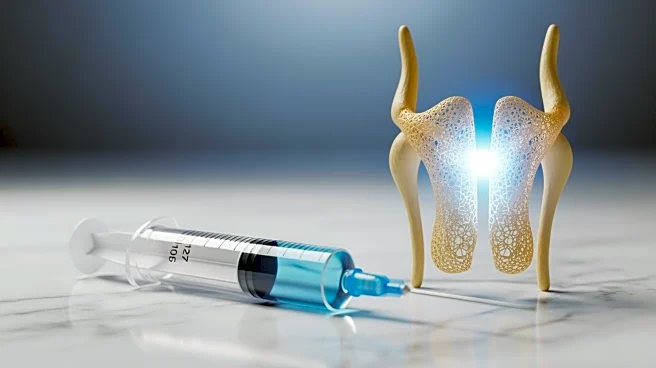
Researchers at Stanford Medicine have developed an injectable treatment that targets a protein linked to aging, successfully restoring cartilage in aging and injured joints. The treatment works by reprogramming
existing cells rather than relying on stem cells. In studies conducted on older mice, the injection not only rebuilt cartilage but also prevented the development of arthritis following knee injuries similar to ACL tears. Human knee tissue collected during joint replacement surgeries also responded positively to the treatment, forming new cartilage that functioned normally. This breakthrough suggests that cartilage lost through aging or arthritis could potentially be restored using localized injections or oral medication, potentially eliminating the need for knee or hip replacement surgery.
Why It's Important?
Osteoarthritis affects approximately one in five adults in the United States, generating an estimated $65 billion in direct healthcare costs annually. Current treatments focus on pain management and joint replacement, as no medication can halt or reverse the disease. The new therapy developed by Stanford Medicine targets the protein 15-PGDH, which becomes more abundant with age and contributes to tissue decline. By inhibiting this protein, the treatment promotes cartilage regeneration without stem cell involvement, offering a promising alternative to existing therapies. This development could significantly impact the healthcare industry by reducing the need for invasive surgeries and improving the quality of life for millions of individuals suffering from joint pain and swelling.
What's Next?
The researchers are hopeful that clinical trials will soon be launched to test the effect of the 15-PGDH inhibitor in cartilage regeneration. Phase 1 clinical trials for muscle weakness have already shown that the inhibitor is safe and active in healthy volunteers. If successful, this treatment could revolutionize the approach to treating osteoarthritis and other age-related joint conditions, potentially leading to widespread adoption in medical practice. The research team is also exploring the broader applications of this therapy in regenerating other tissues affected by aging.
Beyond the Headlines
The implications of this research extend beyond immediate medical applications. By demonstrating a new method of tissue regeneration that does not rely on stem cells, the study challenges existing paradigms in regenerative medicine. This approach could pave the way for further innovations in treating age-related conditions, potentially leading to breakthroughs in other areas such as muscle, nerve, and bone regeneration. The ethical considerations of manipulating gene expression in existing cells also warrant further discussion as this technology advances.