What's Happening?
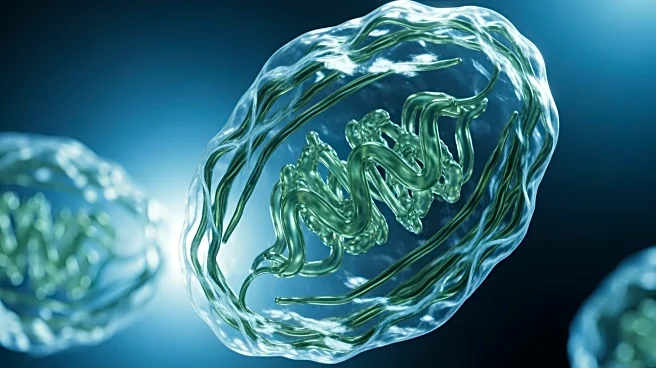
A recent study utilizing cryogenic electron microscopy (Cryo-EM) has revealed insights into how proteins are protected during synthesis, particularly focusing on the glycosylation process. Conducted by
Robert Keenan's group at the University of Chicago and Rajat Rohatgi's lab at Stanford University, the research highlights the role of proteins GRP94 and CCDC134 in preventing hyperglycosylation, which can lead to diseases like osteogenesis imperfecta. The study found that GRP94 recruits CCDC134 and FKBP11 as chaperones to shield it from excessive glycosylation during its formation. This discovery provides a potential pathway for developing targeted drug treatments to disrupt GRP94 without affecting other cellular processes.
Why It's Important?
The findings from this study are crucial for understanding the fundamental processes of protein synthesis and their implications for human health. Glycosylation errors can lead to various diseases, and the ability to regulate this process could have significant therapeutic benefits. The study opens new avenues for drug development, particularly in targeting GRP94-related pathways involved in diabetes and cancer. By understanding how proteins are protected during synthesis, researchers can develop more precise treatments that minimize side effects and improve patient outcomes.
Beyond the Headlines
The study's implications extend beyond immediate medical applications, offering insights into evolutionary biology and cellular mechanisms. The protective role of FKBP11 and CCDC134 suggests an evolutionary adaptation to prevent inappropriate protein interactions, highlighting the complexity of cellular processes. This research also underscores the importance of curiosity-driven science in uncovering fundamental biological mechanisms that can lead to breakthroughs in medicine and biotechnology.