What's Happening?
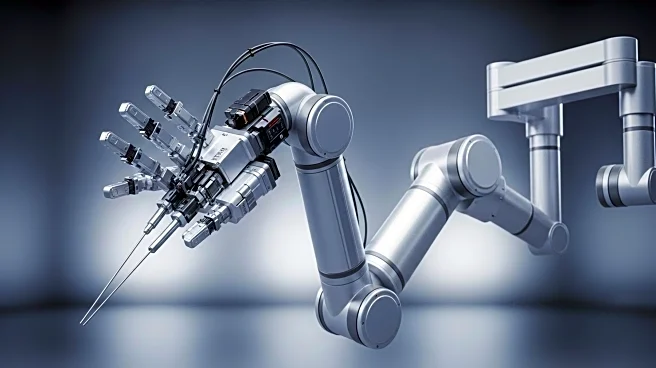
Microbot Medical has announced that its Liberty endovascular surgical robot has received 510(k) clearance from the U.S. Food and Drug Administration (FDA). This clearance marks Liberty as the first FDA-approved single-use, remotely operated robotic system for peripheral endovascular procedures. The company is now positioned to commence commercialization in the U.S., targeting approximately 2.5 million annual peripheral vascular procedures. The Liberty pivotal study demonstrated 100% success in robotic navigation to target areas and reported zero device-related adverse events. Additionally, the study showed a 92% reduction in radiation exposure for physicians. Microbot Medical plans to expand the applications of Liberty to neurovascular, cardiovascular, and peripheral vascular procedures, offering a compact and remotely operable solution.
Why It's Important?
The FDA clearance for Microbot Medical's Liberty robot represents a significant advancement in surgical robotics, potentially transforming the landscape of endovascular procedures. By enabling remote operation, Liberty could enhance procedural precision, safety, and efficiency, addressing critical unmet needs in healthcare. The reduction in radiation exposure is particularly beneficial for physicians, potentially improving their long-term health outcomes. The commercialization of Liberty could lead to cost-effective healthcare solutions, benefiting hospitals and interventional physicians. As the first of its kind, Liberty sets a precedent for future developments in robotic surgery, potentially influencing industry standards and practices.
What's Next?
With FDA clearance secured, Microbot Medical is set to finalize its commercial readiness strategy and begin market entry in the U.S. The company aims to penetrate the peripheral vascular procedure market and explore global opportunities. Microbot Medical is also focusing on adapting Liberty for a wider range of endovascular procedures, supported by a new patent for a modular robotic surgical system. This expansion could further solidify its position in the surgical robotics industry. The upcoming RoboBusiness event in Santa Clara, California, will feature discussions on surgical robotics, including insights into the redesign and launch of next-generation systems.