What is the story about?
What's Happening?
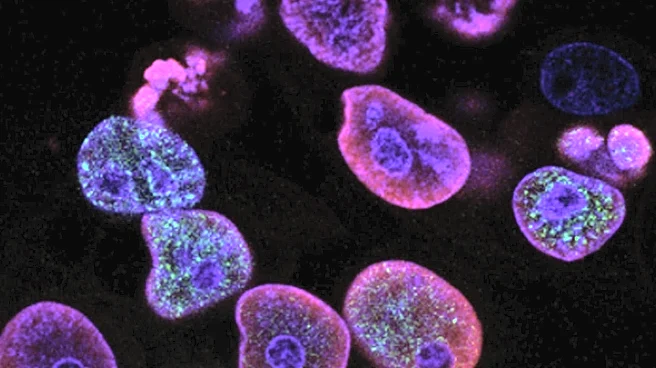
Recent research led by Richard White from Ludwig Oxford and Miranda Hunter from Memorial Sloan Kettering Cancer Center has uncovered how physical stress in the tumor microenvironment can drive cancer cells to become more invasive. Using a zebrafish model of melanoma, the study found that when tumor cells are tightly confined by surrounding tissues, they undergo significant structural and functional changes. This confinement triggers a program of 'neuronal invasion', allowing the cells to migrate and spread into surrounding tissues. A key player in this transformation is HMGB2, a DNA-bending protein that responds to mechanical stress by altering chromatin packaging, thus exposing regions of the genome linked to invasiveness. The study highlights the role of the tumor microenvironment in shaping cancer cell behavior, emphasizing how physical cues can lead to epigenetic changes that make cells more resistant to treatment.
Why It's Important?
The findings from this study are significant as they reveal a previously underappreciated driver of cancer cell behavior: physical stress. Understanding how mechanical forces within the tumor microenvironment can trigger invasive transformations in cancer cells is crucial for developing more effective treatments. Current therapies often target rapidly dividing cells, potentially missing those that have transitioned to a drug-resistant, invasive phenotype. By identifying the factors involved in this switch, researchers hope to develop therapies that can prevent or reverse these transformations, potentially improving outcomes for patients with aggressive cancers like melanoma.
What's Next?
The study suggests that future cancer therapies could focus on targeting the mechanical forces within the tumor microenvironment to prevent or reverse invasive transformations. Researchers may explore ways to disrupt the protective cage-like structure formed by melanoma cells around their nucleus, which involves the LINC complex. Additionally, further studies could investigate how to inhibit HMGB2's role in chromatin remodeling, potentially reducing the invasiveness of cancer cells. These approaches could lead to new treatment strategies that address the flexibility and adaptability of cancer cells in response to their environment.